Abstract
Introduction
High molecular weight kininogen (HK) plays a central role in the kallikrein-kinin system. We previously reported that the cleaved, bradykinin-free form of HK (HKa) induces apoptosis of proliferating endothelial cells and inhibits angiogenesis, and that tumors grow larger and more rapidly in kininogen deficient (mKng1-/-)mice. However, kininogen interacts with many different cell types, and the mechanism by which it inhibits tumor growth in vivo has not been thoroughly characterized. Using mKng1-/- mice we further investigated the role of HKa in angiogenesis and tumor growth, focusing on the effect of kininogen on the tumor stroma and stem cell compartment.
Methods
B16F10 murine melanoma cells (1 x 106) were implanted into syngeneic wild-type and mKng1-/- mice. Tumor size was measured daily using calipers and tumor volume was calculated using the formula V = length x width2 x 0.52. Approximately 17 days after cell implantation, tumors were harvested and processed for flow sorting of live cells, immunohistochemistry, and immunofluorescent staining. Sorted live cells from tumors grown in wild-type and mKng1-/- mice were analyzed by immunoblotting and tumorsphere formation assays, and reimplanted into naïve mice for serial tumor propagation assays.
Results
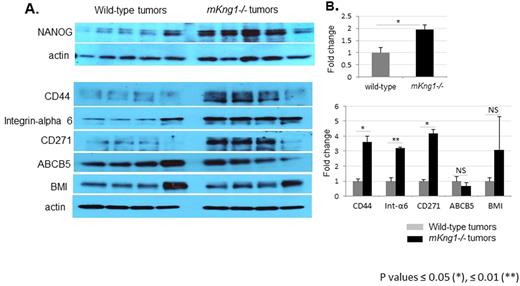
By day 17, tumor volume in mKng1-/- mice was approximately 3-fold larger than in wild-type mice. Multiphoton imaging, CD31 immunostaining and in vivo EdU labeling demonstrated higher vascular density, enhanced angiogenesis and significantly higher proliferation of endothelial cells (2.5 fold) in tumors in mKng1-/- mice compared to those in wild-type mice. Kininogen also affected the cancer stem cell pool, since greater numbers of cells staining positively for cancer stem cell markers, including CD44, NANOG, CD271, BMI, and integrin α6 were detected by immunostaining in tumors from mKng1-/- mice; likewise, increased amounts of these stem cell markers were observed in extracts of tumors from mKng1-/- mice by immunoblotting (see Figure). Though live cells from wild-type or mKng1-/- mice formed similar numbers of tumorspheres when cultured ex vivo in the absence of kininogen, tumorsphere formation from cells from either source was dramatically suppressed by addition of exogenous HKa. To further investigate the role of HKa in tumor initiation we serially passaged tumor cells from wild-type or mKng1-/- mice into tumor-naïve wild-type mice. Two weeks after implantation, the size of tumors resulting from implantation of cells derived from tumors initially grown in mKng1-/- mice was significantly greater than that of tumors arising after implantation of tumor cells initially derived from tumors grown in wild-type mice (743±150 vs. 214±60.9 mm3, respectively). Similar changes in tumor weight were observed (1547±293 vs. 690±133 mg).
Conclusions
While further work is needed to better understand these novel findings, these studies suggest that kininogen not only affects angiogenesis, but the tumor microenvironment, in particular tumor stem cell development and viability.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.